
{cbioportalR} allows you to access cBioPortal’s genomic and clinical data sets directly through R. The package wraps cBioPortal’s API endpoints so R users can easily leverage the existing API to access genomic data on mutations, copy number alterations and fusions as well as data on tumor mutational burden (TMB), microsatellite instability status (MSI) and select clinical data points (depending on the study).
This package was created to work with both the public cBioPortal website, as well as private institutional cBioPortal instances (e.g. MSKCC, GENIE) with appropriate credentials and authentication.
This package is compatible with cBioPortal v5.0, but is subject to change as cBioPortal updates are released. For more information on cBioPortal, see the following publications:
For full documentation on the cBioPortal API, please see the following links:
Note: If you are a MSK researcher working on IMPACT data, you should connect to MSK’s cBioPortal instance to get the most up to date IMPACT data, and you must follow the MSK-IMPACT publication guidelines when using this data
You can install {cbioportalR} with the following code:
install.packages("cbioportalR")Install the development version of {cbioportalR} with:
remotes::install_github("karissawhiting/cbioportalR")Load the package:
library(cbioportalR)If you are using the public domain https://www.cbioportal.org/, you don’t need a token to
start pulling data. If you are using a private instance of cBioPortal
(like MSKCC’s institutional database), you will need to acquire a token
and save it to your .Renviron file (or wherever you store
credentials). Simply log in to your institution’s cBioPortal site,
acquire a token (usually through the ‘Data Access Token’ link in your
username menu in the upper right) and save it in your
.Renviron file. This will save the token as an
environmental variable so you don’t have to hard code the secret key in
your scripts.
Tip: The following {usethis} function can easily find and open
the .Renviron for you:
usethis::edit_r_environ()Paste the token you were given (using the format below) in the .Renviron file and save the file changes. After saving you should restart your R session to ensure the token is saved and recognized.
CBIOPORTAL_TOKEN = 'YOUR_TOKEN'You can test that your token was saved using:
get_cbioportal_token()For every new R session, you need to set your database URL. The
set_cbioportal_db() function will set an environmental
variable for your session that tells the package which database to point
to for all API calls. You can set it to point to the public database
with db = 'www.cbioportal.org' or
db = 'public'. If using a private database you will pass
your institutions cBioPortal URL as db. This function will
both set your URL and check the connection.
set_cbioportal_db(db = "public")
#> ✔ You are successfully connected!
#> ✔ base_url for this R session is now set to "www.cbioportal.org/api"You are now set up for the remainder of your session! API calls depend on your internet connection and possibly a VPN connection so you can use the following to check your connection at any time throughout your session:
test_cbioportal_db()
#> ✔ You are successfully connected!There are many ways to identify and pull data (e.g. by study ID, by sample ID, by molecular profile ID). Having an understanding of how data is organized in cBioPortal will help you determine which functions you need. The figure below outlines the general data schema for cBioPortal and which functions access which levels of the schema:
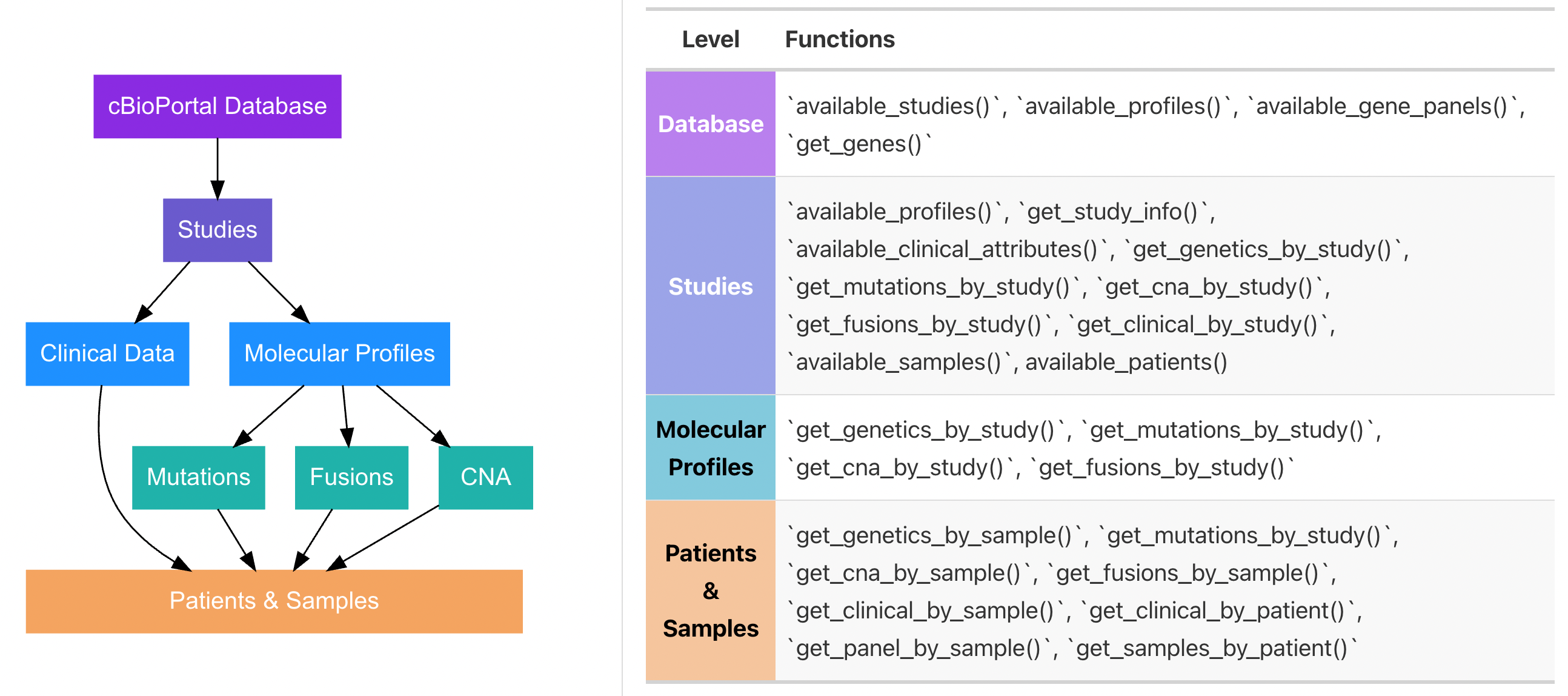
To see available studies in your database you can use:
available_studies() %>%
head(n = 10)
#> # A tibble: 10 × 13
#> studyId name descr…¹ publi…² groups status impor…³ allSa…⁴ readP…⁵ cance…⁶
#> <chr> <chr> <chr> <lgl> <chr> <int> <chr> <int> <lgl> <chr>
#> 1 acc_tcga Adre… "TCGA … TRUE "PUBL… 0 2022-0… 92 TRUE acc
#> 2 bcc_unig… Basa… "Whole… TRUE "PUBL… 0 2022-0… 293 TRUE bcc
#> 3 ampca_bc… Ampu… "Exome… TRUE "PUBL… 0 2022-0… 160 TRUE ampca
#> 4 blca_dfa… Blad… "Whole… TRUE "PUBL… 0 2022-0… 50 TRUE blca
#> 5 blca_msk… Blad… "Compr… TRUE "PUBL… 0 2022-0… 97 TRUE blca
#> 6 blca_bgi Blad… "Whole… TRUE "PUBL… 0 2022-0… 99 TRUE blca
#> 7 blca_msk… Blad… "Genom… TRUE "PUBL… 0 2022-0… 109 TRUE blca
#> 8 all_stju… Hypo… "Whole… TRUE "" 0 2022-0… 44 TRUE myeloid
#> 9 acyc_fmi… Aden… "Targe… TRUE "ACYC… 0 2022-0… 28 TRUE acyc
#> 10 acyc_san… Aden… "Whole… TRUE "ACYC… 0 2022-0… 24 TRUE acyc
#> # … with 3 more variables: referenceGenome <chr>, pmid <chr>, citation <chr>,
#> # and abbreviated variable names ¹description, ²publicStudy, ³importDate,
#> # ⁴allSampleCount, ⁵readPermission, ⁶cancerTypeIdTo view study metadata on a particular study you can use:
get_study_info("acc_tcga") %>%
t()
#> [,1]
#> name "Adrenocortical Carcinoma (TCGA, Firehose Legacy)"
#> description "TCGA Adrenocortical Carcinoma. Source data from <A HREF=\"http://gdac.broadinstitute.org/runs/stddata__2016_01_28/data/ACC/20160128/\">GDAC Firehose</A>. Previously known as TCGA Provisional."
#> publicStudy "TRUE"
#> groups "PUBLIC"
#> status "0"
#> importDate "2022-03-04 17:47:56"
#> allSampleCount "92"
#> sequencedSampleCount "90"
#> cnaSampleCount "90"
#> mrnaRnaSeqSampleCount "0"
#> mrnaRnaSeqV2SampleCount "79"
#> mrnaMicroarraySampleCount "0"
#> miRnaSampleCount "0"
#> methylationHm27SampleCount "0"
#> rppaSampleCount "46"
#> massSpectrometrySampleCount "0"
#> completeSampleCount "75"
#> readPermission "TRUE"
#> studyId "acc_tcga"
#> cancerTypeId "acc"
#> cancerType.name "Adrenocortical Carcinoma"
#> cancerType.dedicatedColor "Purple"
#> cancerType.shortName "ACC"
#> cancerType.parent "adrenal_gland"
#> cancerType.cancerTypeId "acc"
#> referenceGenome "hg19"To pull all genomic data for a particular study you can use:
df <- get_genetics_by_study(study_id = "acc_tcga")
#> ℹ Returning all data for the "acc_tcga_mutations" molecular profile in the "acc_tcga" study
#> ℹ Returning all data for the "acc_tcga_gistic" molecular profile in the "acc_tcga" study
#> ! No "structural_variant" data returned. Error: No molecular profile for `data_type = fusion` found in "acc_tcga". See `available_profiles('acc_tcga')`As a result, you will get a list of data frames with mutation and CNA data respectively. The function will also try to pull fusion (structural variant) data, but there is no fusion data available for this study, as indicated by the function message.
df$mutation %>%
head()
#> # A tibble: 6 × 33
#> hugoG…¹ entre…² uniqu…³ uniqu…⁴ molec…⁵ sampl…⁶ patie…⁷ studyId center mutat…⁸
#> <chr> <int> <chr> <chr> <chr> <chr> <chr> <chr> <chr> <chr>
#> 1 KRT8 3856 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… broad… Somatic
#> 2 LCE1B 353132 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… hgsc.… Somatic
#> 3 SLC9C2 284525 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… broad… Somatic
#> 4 DNAH14 127602 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… broad… Somatic
#> 5 OPN4 94233 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… hgsc.… Somatic
#> 6 DNAJC4 3338 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… hgsc.… Somatic
#> # … with 23 more variables: validationStatus <chr>, tumorAltCount <int>,
#> # tumorRefCount <int>, normalAltCount <int>, normalRefCount <int>,
#> # startPosition <int>, endPosition <int>, referenceAllele <chr>,
#> # proteinChange <chr>, mutationType <chr>, functionalImpactScore <chr>,
#> # fisValue <dbl>, linkXvar <chr>, linkPdb <chr>, linkMsa <chr>,
#> # ncbiBuild <chr>, variantType <chr>, keyword <chr>, chr <chr>,
#> # variantAllele <chr>, refseqMrnaId <chr>, proteinPosStart <int>, …
df$cna %>%
head()
#> # A tibble: 6 × 9
#> hugoGeneSymbol entre…¹ uniqu…² uniqu…³ molec…⁴ sampl…⁵ patie…⁶ studyId alter…⁷
#> <chr> <int> <chr> <chr> <chr> <chr> <chr> <chr> <int>
#> 1 MEOX1 4222 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… 2
#> 2 NUFIP2 57532 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… 2
#> 3 OSBPL7 114881 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… 2
#> 4 TP53I13 90313 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… 2
#> 5 TAOK1 57551 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… 2
#> 6 SPOP 8405 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… 2
#> # … with abbreviated variable names ¹entrezGeneId, ²uniqueSampleKey,
#> # ³uniquePatientKey, ⁴molecularProfileId, ⁵sampleId, ⁶patientId, ⁷alterationYou can also pull data by specific sample IDs but the API requires a
bit more information from you (unlike pulling by study ID which returns
everything available for that study). This can be useful when working
within a very large database or working across samples housed in
multiple different studies. When querying by sample_id you
must also specify the corresponding study_id in which the
samples are housed. When these pieces of information are not provided,
{cbioportalR} makes an informed guess based on your connection and will
throw an informative message to clarify exactly what is being queried.
In the example below, the function defaults to the public version of the
IMPACT database (study_id = "msk_impact_2017").
samples <- available_samples(study_id = "acc_tcga") %>%
pull(sampleId) %>%
head(n = 10)
mutations <- get_mutations_by_sample(sample_id = samples)
#> The following parameters were used in query:
#> Study ID: "msk_impact_2017"
#> Molecular Profile ID: "msk_impact_2017_mutations"
#> Genes: "All available genes"
# no results returned because these samples are not in this study
length(mutations) == 0
#> [1] TRUENo results were returned because the samples are not stored in this
study. When we specify the correct study
(study_id = "acc_tcga"), we get accurate results. You can
check which samples are stored in a study using
available_samples(study_id = "acc_tcga").
mutations <- get_mutations_by_sample(sample_id = samples,
study_id = "acc_tcga")
#> The following parameters were used in query:
#> Study ID: "acc_tcga"
#> Molecular Profile ID: "acc_tcga_mutations"
#> Genes: "All available genes"
mutations %>%
head()
#> # A tibble: 6 × 33
#> hugoG…¹ entre…² uniqu…³ uniqu…⁴ molec…⁵ sampl…⁶ patie…⁷ studyId center mutat…⁸
#> <chr> <int> <chr> <chr> <chr> <chr> <chr> <chr> <chr> <chr>
#> 1 KRT8 3856 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… broad… Somatic
#> 2 LCE1B 353132 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… hgsc.… Somatic
#> 3 SLC9C2 284525 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… broad… Somatic
#> 4 DNAH14 127602 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… broad… Somatic
#> 5 OPN4 94233 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… hgsc.… Somatic
#> 6 DNAJC4 3338 VENHQS… VENHQS… acc_tc… TCGA-O… TCGA-O… acc_tc… hgsc.… Somatic
#> # … with 23 more variables: validationStatus <chr>, tumorAltCount <int>,
#> # tumorRefCount <int>, normalAltCount <int>, normalRefCount <int>,
#> # startPosition <int>, endPosition <int>, referenceAllele <chr>,
#> # proteinChange <chr>, mutationType <chr>, functionalImpactScore <chr>,
#> # fisValue <dbl>, linkXvar <chr>, linkPdb <chr>, linkMsa <chr>,
#> # ncbiBuild <chr>, variantType <chr>, keyword <chr>, chr <chr>,
#> # variantAllele <chr>, refseqMrnaId <chr>, proteinPosStart <int>, …Lastly, you can also pull clinical data or sample metadata (e.g. tumor sample site) by study ID, sample ID or patient ID. To see what data is available, you can use:
available_clinical_attributes(study_id = "acc_tcga") %>%
head()
#> # A tibble: 6 × 7
#> displayName descr…¹ datat…² patie…³ prior…⁴ clini…⁵ studyId
#> <chr> <chr> <chr> <lgl> <chr> <chr> <chr>
#> 1 Diagnosis Age Age at… NUMBER TRUE 1 AGE acc_tc…
#> 2 Neoplasm Disease Stage Americ… The ex… STRING TRUE 1 AJCC_P… acc_tc…
#> 3 American Joint Committee on C… The ve… STRING TRUE 1 AJCC_S… acc_tc…
#> 4 Atypical Mitotic Figures Atypic… STRING TRUE 1 ATYPIC… acc_tc…
#> 5 Cancer Type Cancer… STRING FALSE 1 CANCER… acc_tc…
#> 6 Cancer Type Detailed Cancer… STRING FALSE 1 CANCER… acc_tc…
#> # … with abbreviated variable names ¹description, ²datatype, ³patientAttribute,
#> # ⁴priority, ⁵clinicalAttributeIdget_clinical_by_study("acc_tcga")
#> ! Sample Level Clinical Data: No `clinical_attribute` passed. Defaulting to returning all clinical attributes in "acc_tcga" study
#> ! Patient Level Clinical Data: No `clinical_attribute` passed. Defaulting to returning all clinical attributes in "acc_tcga" study
#> # A tibble: 6,292 × 6
#> uniquePatientKey patientId studyId clinicalAt…¹ value dataL…²
#> <chr> <chr> <chr> <chr> <chr> <chr>
#> 1 VENHQS1PUi1BNUoxOmFjY190Y2dh TCGA-OR-A5J1 acc_tcga AGE 58 PATIENT
#> 2 VENHQS1PUi1BNUoxOmFjY190Y2dh TCGA-OR-A5J1 acc_tcga AJCC_PATHOL… Stag… PATIENT
#> 3 VENHQS1PUi1BNUoxOmFjY190Y2dh TCGA-OR-A5J1 acc_tcga ATYPICAL_MI… Atyp… PATIENT
#> 4 VENHQS1PUi1BNUoxOmFjY190Y2dh TCGA-OR-A5J1 acc_tcga CAPSULAR_IN… Inva… PATIENT
#> 5 VENHQS1PUi1BNUoxOmFjY190Y2dh TCGA-OR-A5J1 acc_tcga CLIN_M_STAGE M0 PATIENT
#> 6 VENHQS1PUi1BNUoxOmFjY190Y2dh TCGA-OR-A5J1 acc_tcga CT_SCAN_PRE… [Unk… PATIENT
#> 7 VENHQS1PUi1BNUoxOmFjY190Y2dh TCGA-OR-A5J1 acc_tcga CYTOPLASM_P… Cyto… PATIENT
#> 8 VENHQS1PUi1BNUoxOmFjY190Y2dh TCGA-OR-A5J1 acc_tcga DAYS_TO_INI… 0 PATIENT
#> 9 VENHQS1PUi1BNUoxOmFjY190Y2dh TCGA-OR-A5J1 acc_tcga DFS_MONTHS 24.77 PATIENT
#> 10 VENHQS1PUi1BNUoxOmFjY190Y2dh TCGA-OR-A5J1 acc_tcga DFS_STATUS 1:Re… PATIENT
#> # … with 6,282 more rows, and abbreviated variable names ¹clinicalAttributeId,
#> # ²dataLevelget_clinical_by_sample(sample_id = samples, study_id = "acc_tcga") %>%
head(10)
#> ! No `clinical_attribute` passed. Defaulting to returning
#> all clinical attributes in "acc_tcga" study
#> # A tibble: 10 × 7
#> uniqueSampleKey uniqu…¹ sampl…² patie…³ studyId clini…⁴ value
#> <chr> <chr> <chr> <chr> <chr> <chr> <chr>
#> 1 VENHQS1PUi1BNUoxLTAxOmFjY190Y2… VENHQS… TCGA-O… TCGA-O… acc_tc… CANCER… Adre…
#> 2 VENHQS1PUi1BNUoxLTAxOmFjY190Y2… VENHQS… TCGA-O… TCGA-O… acc_tc… CANCER… Adre…
#> 3 VENHQS1PUi1BNUoxLTAxOmFjY190Y2… VENHQS… TCGA-O… TCGA-O… acc_tc… DAYS_T… 4691
#> 4 VENHQS1PUi1BNUoxLTAxOmFjY190Y2… VENHQS… TCGA-O… TCGA-O… acc_tc… FRACTI… 0.05…
#> 5 VENHQS1PUi1BNUoxLTAxOmFjY190Y2… VENHQS… TCGA-O… TCGA-O… acc_tc… IS_FFPE NO
#> 6 VENHQS1PUi1BNUoxLTAxOmFjY190Y2… VENHQS… TCGA-O… TCGA-O… acc_tc… MUTATI… 39
#> 7 VENHQS1PUi1BNUoxLTAxOmFjY190Y2… VENHQS… TCGA-O… TCGA-O… acc_tc… OCT_EM… TRUE
#> 8 VENHQS1PUi1BNUoxLTAxOmFjY190Y2… VENHQS… TCGA-O… TCGA-O… acc_tc… ONCOTR… ACC
#> 9 VENHQS1PUi1BNUoxLTAxOmFjY190Y2… VENHQS… TCGA-O… TCGA-O… acc_tc… OTHER_… E403…
#> 10 VENHQS1PUi1BNUoxLTAxOmFjY190Y2… VENHQS… TCGA-O… TCGA-O… acc_tc… PATHOL… TCGA…
#> # … with abbreviated variable names ¹uniquePatientKey, ²sampleId, ³patientId,
#> # ⁴clinicalAttributeIdpatients <- available_patients(study_id = "acc_tcga") %>%
pull(patientId) %>%
head(n = 10)
get_clinical_by_patient(patient_id = patients, study_id = "acc_tcga",
clinical_attribute = "AGE") %>%
head(10)All functions that pull by study IDs are limited to pulling data from
one study at a time. If you need to pull specific samples from multiple
studies, you likely want to pull by sample ID (instead of study ID) and
supply the function with a dataframe of sample_study_pairs
that specify where the function should look for each study. For more
information see the Overview
of Workflow Vignette.
Please note that {cbioportalR} is released with a Contributor Code of Conduct. By contributing to this project, you agree to abide by its terms.
Thank you to contributors!
@arorarshi, @AxelitoMartin, @edrill, @jalavery, @ddsjoberg @karomanchuk @hfuchs5 @alrein-05
Thank you Isaak Liptzin for the hex sticker!